Breaking news
Hot topics
The editorial team at Floretina.com is committed to select the news of the month. We will interview established physicians, as well as young ophthalmologists, scientific advisors and Industry representatives. Register on the website to receive news notifications!
Search in Hot Topic section
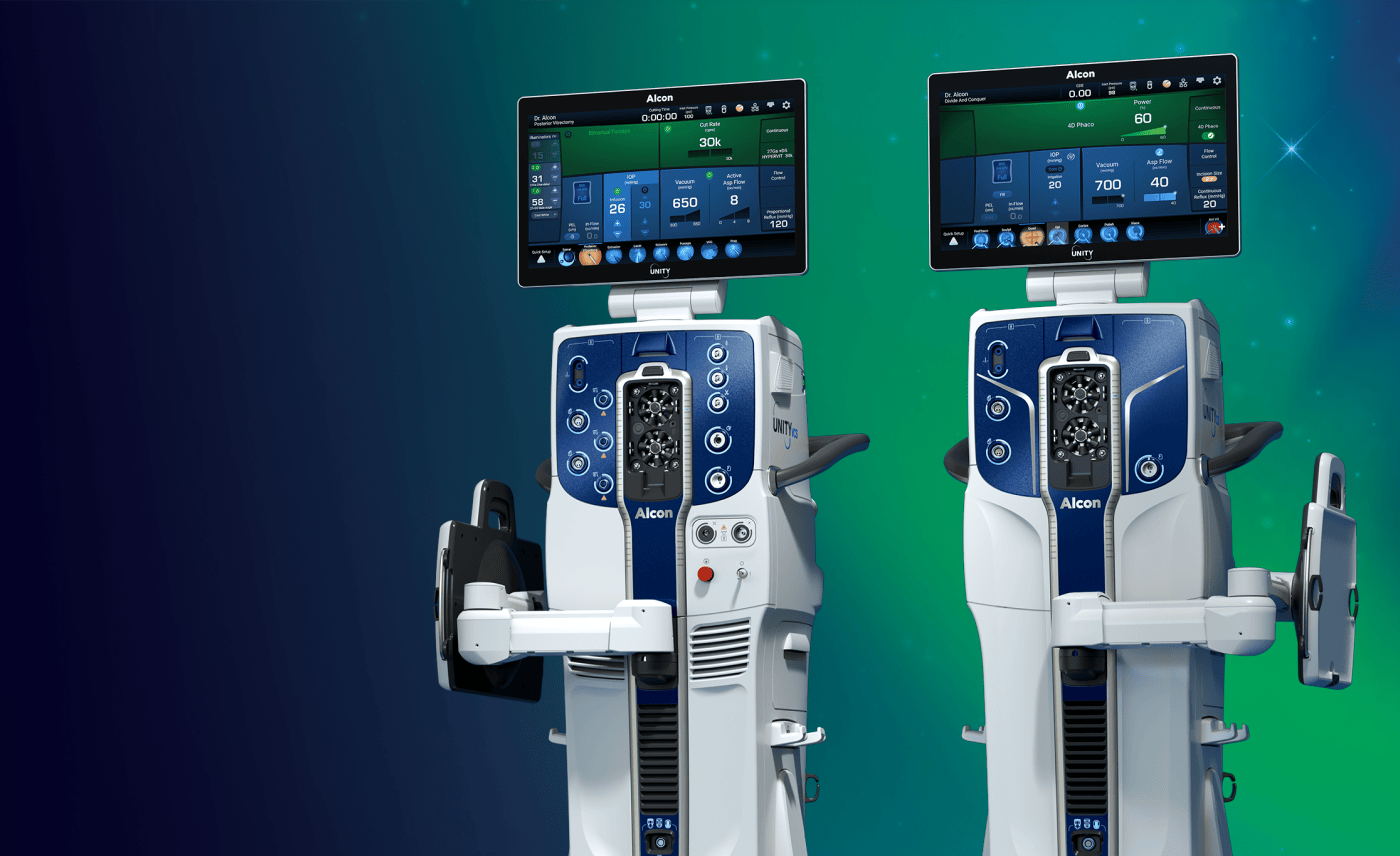
Superior Efficiency in Action: Surgeons Share Their Experience with Alcon Unity VCS
Alcon, the global leader in eye care, will showcase its latest innovation for cataract and vitreoretinal surgery at the FLORetina-ICOOR 2025 Congress in Florence, Italy, December 4-7. Floretina delegates are invited to attend live surgery sessions and visit the Alcon booth to find out more about UNITY® VCS, a versatile platform that offers two configurations: the combined Vitreoretinal Cataract System (VCS) and the standalone Cataract System (CS). Read more
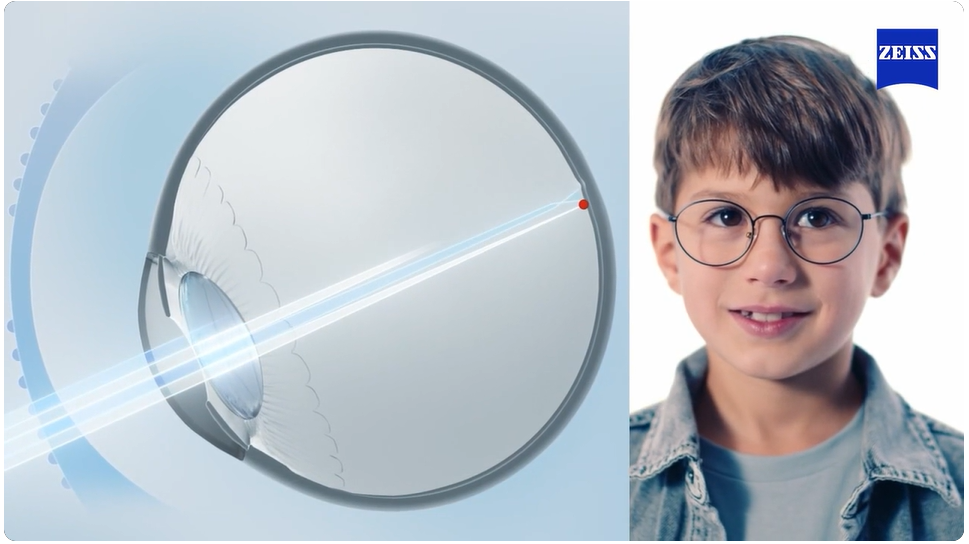
ZEISS MyoCare update results at ARVO 2025 confirm sustained efficacy
After satisfactory results provided last year, ZEISS presented an update for their two major on-going multicentre clinical trials for MyoCare lenses on both Asian and Caucasian children at this year's ARVO meeting in Salt Lake City, USA demonstrating further their continuing efficacy to slow the progression of myopia in children. Read more
The quantum leap
Purpose: Key opinion leaders in retina on the trial results of Eylea HD (Aflibercept 8mg) in terms of safety and efficacy. Read more
ZEISS MyoCare lenses: updating progress
While the development of strategies and innovation for the prevention of myopia and high myopia in children is gaining momentum in the post pandemic world, there is still a lack of data regarding the effectiveness of these methods across different FLORETINA BREAKING NEWS ZEISS MyoCare: updating progress ethnic groups and with larger samples that could provide a more robust body of evidence. Read more
ZEISS MyoCare: focusing ideas
The ongoing global growth of myopia is reaching worrisome proportions. As shown already in a 2016 study by Holden et al, the observed trend and prevalence of eastern Asia is already indicating nearsightedness as one of the candidates to the role of pandemic disease. Read more
AMD, challenging the unmet needs
The introduction of anti-VEGF compounds more than a decade ago represented a paradigm shift of undisputable magnitude. Nevertheless, many unmet needs in the management of age-related macular degeneration still remain. Read more
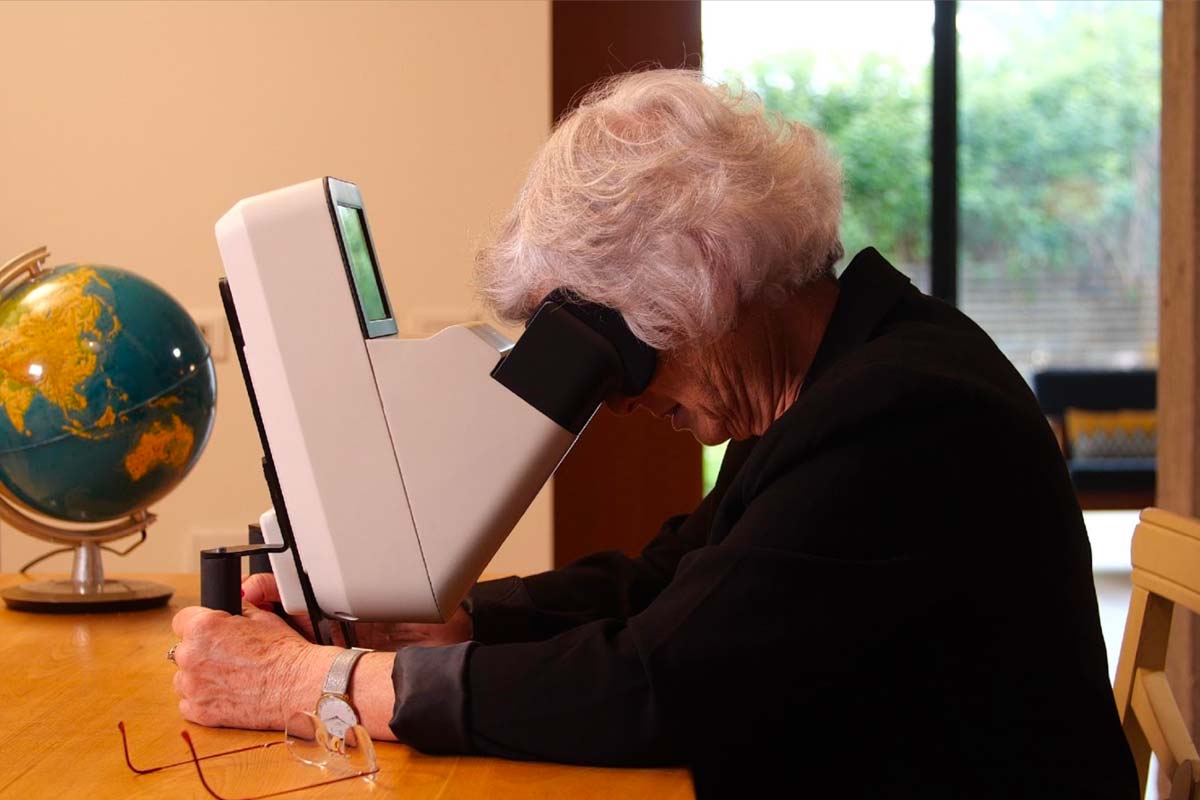
Home Monitoring, a pathway to personalisation
A new idea to bridge the gap between patient and physician: among the most recent innovations in ophthalmology, and retina in particular, the development of home monitoring systems shows potential to improve efficacy of treatment and care. Read more
Post hoc analysis of trial data shows rapid DR progression In untreated fellow eyes
Untreated fellow eyes of patients with moderately severe to severe nonproliferative diabetic retinopathy (NPDR) are at risk of rapid disease progression and vision loss, according to researchers from the Mayo Clinic of Rochester, Minnesota. Read more
A new biomarker for progression of geographic atrophy can be found in choroidal vascularity index, study shows.
Choroidal vascularity index (CVI) impairment is strictly related to the progression of geographic atrophy (GA) and could be considered a predictor of disease progression, as well as a new potential biomarker for the evaluation of treatment efficacy, a clinical trial shows. Read more
Positive Phase III topline results of novel eye drop candidate for the treatment of presbyopia paves the way to regulatory submission in the U.S.
Following successful Phase 2b results, a novel eye drop candidate for the treatment of presbyopia achieved topline results in the NEAR-1 and NEAR-2 Phase III clinical trials, according to a press release by Orasis Pharmaceuticals. Read more
EC approves Brolucizumab for the treatment of DME
The European Commission has granted approval to Beovu® 6mg (brolucizumab, Novartis) for the treatment of visual impairment related to diabetic macular oedema. Read more
Zeiss receives U.S. FDA clearance for Quatera 700 phaco technology
The U.S. Food and Drug Administration has granted clearance to Quatera 700 from Zeiss, a complete and digitally-integrated phacoemulsification system designed to optimise workflow efficiency from the clinic to the operating theatre. Read more
Use of phosphodiesterase type 5 inhibitors may be associated with severe ocular adverse effects
Regular use of phosphodiesterase type 5 inhibitors (PDE5Is) was associated with an increased risk for serous retinal detachment (SRD), ischemic optic neuropathy (ION) and retinal vascular occlusion (RVO) in a large cohort study Read more
Global survey sheds light on geographic atrophy’s heavy toll on life quality, calls for broader disease education
A global survey conducted by the Harris Poll found that living with geographic atrophy (GA) causes a substantial emotional burden and impact on independence. Read more
Joint effort with the University of Leicester identifies multiple gene impact in rare sight disorder
For the first time, genetic defects influencing the spectrum of vision development and causing problems in developing babies’ eyes have been described by an international team of health researchers. Read more
Phase IV study aims at investigating safety and efficacy of Vabysmo with a focus on underrepresented population
Genentech has launched a new Phase IV, multicenter, open-label, single-arm clinical trial specifically designed to study Vabysmo™ (faricimab-svoa) in underrepresented populations affected by diabetic macular edema. Read more
Ziemer latest femtosecond laser receives FDA clearance
Ziemer Ophthalmic Systems has received FDA clearance for FEMTO Z8 NEO, the successor of Femtosecond Laser LDV Z8, as announced by the company on April 22. Read more